Study of diffusion.
T.Tokoro in University of
Windsor
The rate of absorption was found to be linear with t0.5 and consistent with the equation developed by Crank [1] for the case where diffusion of a liquid into a solid matrix is driven by a concentration gradient, and there are no chemical interactions between the liquid and the matrix. The theoretical curve for this model is:
M(t)/M = 4(D/l2)0.5t0.5[(1/p) 0.5 + 2n=1 to (-1)nierfc{(nl)/(2(Dt) 0.5)}]. (1)
In this equation, M(t) and M are the weight increase due to the absorption at time t and at very long times, D is the diffusion coefficient, and l is the thickness of the polymer solid. Absorption is occurred from both sides of the sample. The values for the diffusion coefficients were calculated from the initial linear portions of the experimental curves using following equations, which follows from equation (1) for the early stages of diffusion [1],
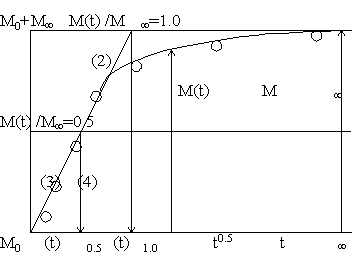
M(t)/M = 4{D/(pl2)}0.5t0.5. (2)
D = (p/64) (l2/t)0.5 = 0.049 (l2/t)0.5 (3)
D = (p/16) (l2/t)1.0 = 0.196 (l2/t)1.0 (4)
where (l2/ t)0.5 and (l2/ t)1.0 means the (l2/t) at the time M(t)/M = 0.5 and 1.0, respectively.
Therefore, to determine the diffusion coefficient M value has to be known. Equation (4) is used in [2] and relation of (2) to (4) are shown schematically in Fig.1. Whiles, sometime this value M is misused by M0, the initial mass of polymer solid [3]. In some cases the thickness l is also misunderstood [4]. They used l/2 instead of l because both sides of solid absorb the liquid and therefore they think l/2 is the thickness for diffusion. If using this half of the thickness 4 in (1) should be change to 2. This is original situation of [1] to introduce (1) theoretically.
Using M provides the correct value
of diffusion coefficient of liquid. However, using M0
instead of M gives us how amount of liquid is absorbed
during the immersion time of t in weight percent. Therefore, this
is also useful to estimate the absorbed amount of liquid in weight
percent at the time t. In other words, using M not
depends on the density of polymer (g/cm3) but using
M0 depends on it.
[1] J. Crank, "Mathematics of Diffusion," 2nd Edition, Claredon, Oxford, 1975.
[2] S.L. Rice and A.F.Diaz, "Absorption of Silicone Oil by a Dimethylsiloxane Elastomer", Rubber Chemistry and Technology, Vol.61, pp.194-204, 1988.
[3]R.S.Gorur, J.W.Chang and O.G.Amburgey, "Surface Hydrophobicity of Polymers Used for Outdoor Insulation", IEEE Trans. PD., Vol.5, No.4, pp.1923-1933, 1990.
[4] H. Deng and R. Hackam, .
